Good Manufacturing Practice GMP Certification Process in Nepal
In the rapidly evolving global marketplace, ensuring product quality, safety, and compliance with regulatory standards is paramount for businesses across various industries. Good Manufacturing Practices (GMP) certification is one crucial certification that helps organizations accomplish these objectives. The article analyzes GMP certification, its process, standards, and implementation in different areas, including Nepal.
What is Good Manufacturing Practices (GMP)?
Good Manufacturing Practices (GMP) is a set of guidelines and regulations established by regulatory bodies to make sure that products are consistently produced and controlled as per quality standards.
GMPs get designed to minimize the risks associated with product manufacturing that can affect quality, safety, and efficacy. By implementing GMP principles, organizations can ensure manufacturing processes are reliable, well-documented, and compliant with regulatory requirements.
GMP Certification Process
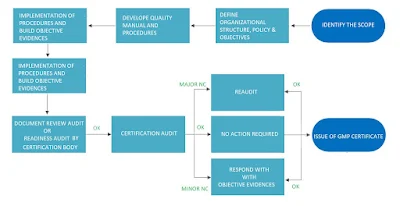
Obtaining GMP certification involves several key steps that organizations must follow. A robust quality management system that adheres to GMP guidelines must be developed and implemented. This process includes establishing standard operating procedures (SOPs), training employees, and conducting internal audits to identify areas for improvement and ensure compliance.
After internal systems are in place, organizations can engage with a certification body accredited to grant GMP certifications. The certification body will extensively audit the organization's quality-assured facilities, laboratory practice, processes, and documentation to assess compliance with GMP standards. The audit process typically includes a comprehensive review of quality management systems, GMP training, personnel training records, manufacturing procedures, equipment calibration, cleanliness, and documentation practices.
During the audit, the certification body evaluates the organization's ability to consistently produce products that meet quality standards. Successful completion of the audit leads to the issuance of GMP certification. This auditing demonstrates that the organization has completed the requirements for manufacturing safe and high-quality products.
GMP Documentation and Recordkeeping
Documentation and recordkeeping play a crucial role in GMP compliance. Organizations must maintain accurate and comprehensive records of all manufacturing and quality control activities. This documentation includes batch records, standard operating procedures, specifications, validation protocols, change control documentation, and any deviations or corrective actions taken.
Proper documentation ensures traceability, facilitates audits and inspections, and enables organizations to identify and rectify deviations or non-compliance promptly. It is essential to establish robust documentation practices that include clear instructions, detailed procedures, and accurate recordkeeping to demonstrate compliance with GMP guidelines.
GMP Auditing and Inspection
Regular audits and inspections get conducted to verify GMP compliance. Regulatory bodies, certification bodies, or independent third-party auditors typically perform these assessments. The purpose of audits and inspections is to ensure that organizations adhere to GMP guidelines and maintain consistent quality practices.
Auditors review various aspects of the organization's operations during an audit, including the quality management system, facilities, equipment, processes, and documentation. They assess cleanliness, medical devices, personnel hygiene, equipment calibration, validation protocols, and adherence to standard operating procedures. The auditors provide feedback on areas that require improvement and help organizations identify opportunities for enhancing their quality systems.
Corrective and Preventive Actions (CAPA)
The GMP framework emphasizes implementing Corrective and Preventive Actions (CAPA). When deviations or non-compliance get identified during audits or inspections, organizations must take immediate corrective actions to address the issue and prevent recurrence. This introduction to good manufacturing action may involve investigating the root cause of the deviation, implementing corrective measures, and evaluating their effectiveness.
CAPA procedures are crucial for maintaining and improving product quality. They demonstrate an organization's commitment to continuous improvement and help prevent the recurrence of non-compliance issues. Organizations can mitigate risks and enhance their quality management systems by addressing deviations promptly and implementing preventive measures.
GMP Certification Standards and Guidelines
GMP certification standards vary across countries and industries but align with internationally recognized guidelines. These guidelines provide a framework for ensuring product quality, safety, and efficacy. In the pharmaceutical industry, GMP standards often get based on guidelines set by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the World Health Organization (WHO).
The WHO's GMP guidelines provide comprehensive guidance on implementing quality management systems for pharmaceutical manufacturing. These guidelines cover various aspects, including personnel, premises, quality-assured equipment or medical devices, documentation, production, quality control, storage, regulatory compliance, and distribution. Adhering to these guidelines helps organizations ensure that their pharmaceutical products meet the required quality standards and are safe for use.
The ICH, a global organization that brings together regulatory authorities and the pharmaceutical industry, has developed a series of guidelines known as the ICH Quality Guidelines. These guidelines provide recommendations on various quality aspects, including pharmaceutical manufacturing and development, quality risk management, and the control of impurities. They are a valuable resource for organizations searching to align their quality practices with internationally recognized standards.
Implementing GMP in Different Areas
GMP principles can get applied across various organizational areas to ensure consistent quality practices throughout the product lifecycle. Let's explore a few key areas where GMP implementation is crucial:
- GMP for Quality Control and Testing
Quality control and testing are vital components of GMP. Organizations must establish rigorous quality control measures during manufacturing, testing, and release of products to maintain quality standards. This quality control involves establishing appropriate testing methods, conducting regular quality checks, and documenting the results accurately.
Quality control laboratories play a critical role in GMP compliance. These laboratories must be well-equipped, staffed with trained personnel, and follow standardized procedures for testing raw materials, intermediates, and finished products. By adhering to GMP principles in quality control and testing, organizations can ensure that their products meet the required specifications and are safe for consumers.
- GMP for Supply Chain Management
Supply chain management is another crucial area where GMP principles should get implemented. It involves managing the flow of materials, components and finished products from suppliers to customers while ensuring quality and compliance at every step.
Organizations must establish controls and audits to ensure that raw materials and components used in manufacturing meet the required quality standards. This management includes conducting supplier evaluations, implementing quality agreements with suppliers, and regularly auditing their facilities and processes.
Proper storage and transportation practices are essential to maintain product quality throughout the supply chain. Organizations must ensure that storage conditions, such as temperature and humidity, are suitable for storing products. Additionally, proper labeling, identification, and tracking of all pharmaceutical manufacturing materials are necessary to prevent mix-ups or contamination.
By implementing GMP practices in supply chain management, organizations can reduce the risks associated with poor-quality materials, minimize product recalls, and enhance customer satisfaction.
- GMP for Packaging and Labeling
Packaging and labeling ensure product integrity, safety, and regulatory compliance. GMP principles should get applied to packaging and labeling processes to prevent mix-ups, misbranding, and contamination.
Organizations must establish controls over packaging materials, including their storage, handling, and usage. This labeling involves verifying the quality and suitability of packaging materials, conducting regular inspections, and maintaining proper documentation.
Labeling accuracy is essential for learning management systems to provide consumers with accurate information about the product, its usage, and potential risks. GMP guidelines emphasize the importance of proper labeling practices to ensure that products get identified along with all required information.
By implementing GMP practices in packaging and labeling, organizations can ensure that their products are adequately packaged, labeled, and safe for use by consumers.
Case Studies and Best Practices of GMP in Nepal
Examining case studies and best practices can provide valuable insights into solution overview of GMP practices' successful implementation and their impact on organizations. Below are a few examples of organizations that have embraced GMP certification and achieved notable results:
Case Study 1: Himalayan Herbal Products
Himalayan Herbal Products is a Nepalese company producing herbal medicines and supplements. With a vision to provide high-quality herbal products to both local and international markets, the company recognized the importance of GMP certification to ensure the safety and efficacy of its products.
While achieving GMP certification, Himalayan Herbal Products overhauled its manufacturing processes and quality management systems. The company implemented quality control measures, updated equipment to meet GMP standards, and upgraded facilities to meet GMP requirements.
The company also prioritized employee training and development to ensure a thorough understanding of GMP principles and best practices. Training sessions got conducted to enhance employees' knowledge of GMP guidelines, documentation practices, and quality control procedures.
Himalayan Herbal Products underwent a rigorous GMP audit by an accredited certification body. The audit assessed their compliance with GMP standards, including manufacturing processes, documentation practices, quality control procedures, and facility conditions. The company successfully met all requirements and obtained GMP certification.
The impact of GMP certification was significant for Himalayan Herbal Products. The certification enhanced their credibility and reputation and opened doors to international markets. With GMP training certification, the company expanded its customer base and gained the trust of distributors and consumers looking for safe, high-quality herbal products. This success story demonstrates the positive impact of GMP certification on Nepalese companies and enables them to compete globally and contribute to the country's herbal industry.
Case Study 2: Everest Pharmaceutical Industries
Among Nepal's top pharmaceutical companies is Everest Pharmaceutical Industries. The organization recognized the importance of GMP certification to ensure the quality and safety of its pharmaceutical products. This certification was to maintain its competitive edge in the market.
Everest Pharmaceutical Industries implemented measures to improve its manufacturing processes and quality management systems to achieve GMP certification. They thoroughly evaluated their existing procedures and identified areas that required enhancement. These procedures included establishing robust documentation practices, implementing standardized operating procedures, and upgrading their facilities to meet GMP standards.
Employee training and development got prioritized to ensure a deep understanding of GMP principles and practices. Training programs got designed to educate employees on GMP guidelines, quality control procedures, and the importance of adhering to regulatory requirements.
The organization also focused on continuous improvement, regularly conducting internal audits and assessments to identify areas for enhancement and ensure ongoing compliance with GMP standards.
After implementing these measures, Everest Pharmaceutical Industries underwent a comprehensive GMP audit conducted by an accredited certification body. The audit assessed their compliance with GMP guidelines, including quality management systems, manufacturing processes, documentation practices, personal hygiene, and quality control procedures. The organization successfully obtained GMP certification, showcasing its commitment to maintaining high-quality standards.
GMP certification had a significant impact on Everest Pharmaceutical Industries. It improved the company's reputation and credibility among healthcare professionals, regulators, and consumers. The certification opened doors to new business opportunities and facilitated the export of their pharmaceutical products to international markets. Furthermore, GMP practices helped streamline operations, enhance product quality, and ensure patient safety.
These case studies from Nepal demonstrate the final assessments of positive outcomes of implementing GMP practices. They highlight how GMP certification can contribute to the growth and success of companies in the pharmaceutical and herbal industries, enabling them to provide safe and high-quality products while gaining a competitive advantage in domestic and international markets.
Best Practices of GMP in Nepal:
- Continuous Improvement and Training
An essential best practice in maintaining GMP certification is the commitment to continuous improvement and employee training. Organizations prioritizing continuous improvement strive to enhance their quality management systems, manufacturing processes, and documentation practices.
Regular internal audits and self-assessments play a vital role in identifying areas for improvement. Organizations can proactively address deviations, implement corrective actions, and prevent recurrence by conducting thorough operations reviews. These internal audits also ensure that employees remain vigilant and proactive in adhering to GMP guidelines.
Employee training and development are integral components of maintaining GMP compliance. Organizations should provide regular training sessions to update employees on GMP principles, regulatory changes, and best practices. Training programs should get tailored to specific job roles and responsibilities, ensuring employees understand their roles in maintaining GMP compliance.
Training programs can cover various topics, including GMP principles, documentation practices, hygiene standards, and quality control procedures. By investing in employee training, organizations consistently empower their workforce to follow GMP guidelines, contribute to continuous improvement efforts, and raise quality standards.
- Supplier Quality Management
Maintaining solid relationships with suppliers and implementing robust supplier quality management processes is a critical best practice for organizations seeking GMP certification. Raw materials and components used in manufacturing can significantly impact product quality. Therefore, it is essential to ensure that suppliers meet stringent quality standards.
Organizations should establish a thorough supplier evaluation process to assess potential suppliers' capabilities and quality systems. This evaluation may include site visits, quality audits, and assessments of their documentation practices. Organizations can minimize the risks of poor-quality materials by selecting reliable and GMP-compliant suppliers.
Quality agreements with suppliers effectively ensure mutual understanding and compliance with GMP requirements. These agreements outline the quality expectations, responsibilities, and communication channels between the organization and its suppliers. They serve as a reference documents to maintain consistent quality standards throughout the supply chain.
Regular supplier audits are essential to monitor and maintain compliance with GMP guidelines. Organizations should conduct periodic audits to assess supplier performance, identify deviations or non-compliance, and collaborate with suppliers to implement corrective actions.
By implementing robust supplier quality management processes, organizations can minimize all the risks associated with poor-quality materials, enhance product quality, and maintain GMP compliance.
The journey toward GMP certification involves the following:
- Comprehensive assessments.
- Upgrades to facilities and equipment.
- Implementation of standardized operating procedures.
- Establishment of rigorous documentation practices.
Additionally, employee training programs ensure that all staff members get equipped with the knowledge and understanding of GMP principles and best practices.
Continuous improvement plays a vital role in maintaining GMP certification. Internal audits and assessments help identify areas for enhancement and ensure ongoing compliance with GMP guidelines. Organizations can consistently meet and exceed quality standards by prioritizing continuous improvement, thus enhancing their products' safety and efficacy.
GMP certification has proven valuable for Nepalese companies, enabling them to contend globally and contribute to the country's thriving industries. It serves as a mark of excellence and assures consumers, healthcare professionals, and regulatory authorities that products manufactured by certified companies meet the highest quality standards.
As Nepal continues strengthening its position in various sectors, GMP certification catalyzes growth and development. By embracing GMP practices, Nepalese companies are poised to establish themselves as trusted providers of safe and high-quality products, further bolstering the country's economy and reputation in the global marketplace.
Conclusion
GMP certification has emerged as crucial in ensuring Nepalese companies' product quality, safety, and compliance. Organizations such as Himalayan Herbal Products and Everest Pharmaceutical Industries have experienced significant benefits and success by implementing robust practices, employee training, and continuous improvement efforts.
GMP certification serves as a testament to the commitment of these organizations to maintain high standards of manufacturing and quality control. It has enhanced its reputation and opened doors to new markets domestically and internationally. With GMP certification, these companies have gained a competitive advantage, as consumers and regulatory authorities recognize the importance of adherence to internationally recognized quality standards.
Frequently Asked Questions:
Why is GMP important?
GMP, which stands for Good
Manufacturing Practice, is important because it ensures that products are
consistently produced and controlled according to established quality
standards. By adhering to GMP guidelines, organizations can minimize the risks
involved in the manufacturing process, guarantee product safety, and maintain
their reputation for delivering high-quality goods.
Why is GMP important in the pharmaceutical industry?
GMP is of utmost importance in the
pharmaceutical industry because the products manufactured here directly impact
human health. Adhering to GMP regulations ensures that pharmaceutical companies
maintain the highest standards in manufacturing, packaging, labeling, and
testing of drugs and medical products. This ensures the safety and efficacy of
medicines for patients.
How to get GMP certified?
To get GMP certified, organizations, including pharmaceutical companies, must follow the guidelines set by the regulatory authorities in their respective countries or regions. The process typically involves self-assessment, implementation of GMP principles, conducting internal audits, and making necessary improvements to comply with the standards. Once the organization is ready, they can apply for GMP certification from accredited certification bodies that specialize in GMP audits.
What is GMP in the food industry?
In the food industry, GMP refers to Good Manufacturing Practice. It involves adherence to guidelines and regulations to ensure that food products are consistently produced, controlled, and processed in a safe and hygienic manner. GMP in the food industry ensures that food is of high quality, free from contaminants, and safe for consumption.
How do industries acquire WHO GMP certification in Nepal?
To acquire WHO GMP certification
in Nepal, pharmaceutical companies and other industries involved in the
production of medicinal products must comply with the World Health
Organization's Good Manufacturing Practice guidelines. They need to undergo a
rigorous evaluation of their manufacturing processes and facilities by
recognized certification bodies or government agencies to demonstrate
compliance with WHO GMP standards.
How to check if a GMP certificate is valid?
To check the validity of a GMP
certificate, you should verify it with the organization or certification body
that issued the certificate. Typically, you can do this by contacting the
certification body directly and providing them with the relevant certificate
details for verification.
What does GMP in coffee mean?
GMP in the context of coffee
stands for Good Manufacturing Practice. It refers to the guidelines and
standards that ensure the safe and hygienic production, processing, and
packaging of coffee products. GMP in the coffee industry aims to maintain the
quality, purity, and safety of coffee for consumers.
What does GMP stand for in business?
In business, GMP stands for Good Manufacturing Practice. It encompasses the processes, procedures, and quality standards that businesses implement to ensure consistent and high-quality production of goods. GMP in business is crucial to meet customer expectations, comply with industry regulations, and maintain a positive brand reputation.
In order to maintain a seamless and efficient ISO
certification process, partnering with a trusted ISO consultant is crucial. At
Quality Management System Nepal Pvt.Ltd., we are committed to providing your
organization with expert guidance and support, ensuring a cost-effective and
successful ISO implementation journey. As the leading ISO System Certification
body in Nepal, we offer a comprehensive range of certification services
tailored to your organization's needs.
Explore our range of ISO certification services:
- ISO 55001 Certification in Nepal: Enhance asset
management and energy efficiency.
- TL 9000 Certification in Nepal: Elevate quality
standards in the telecommunications industry.
- IATF 16949 Certification in Nepal: Elevate automotive
quality and meet industry requirements.
- ISO 37001 Certification in Nepal: Strengthen
anti-bribery management systems.
- AS 9001 Certification in Nepal: Attain aerospace quality
standards for excellence.
- ISO FSSC 22000 Certification in Nepal: Ensure safe and
quality food production.
- ISO 29990 Certification in Nepal: Elevate training and
learning services.
- ISO SA 8000 Certification in Nepal: Prioritize social
accountability and ethical practices.
- ISO 27017 Certification in Nepal: Ensure secure cloud
computing environments.
- ISO 20000-1:2018 Certification in Nepal: Elevate IT
service management systems.
- ISO 22301 Certification in Nepal: Enhance business
continuity and resilience.
- ISO 41001 Certification in Nepal: Optimize facility
management systems.
- HACCP Certification in Nepal: Ensure food safety and
hazard analysis.
- ISO 50001 Certification in Nepal: Enhance energy
management and efficiency.
- ISO 13485 Certification in Nepal: Elevate medical device
quality standards.
- Good Manufacturing Practice (GMP) Certification in Nepal:
Ensure quality and compliance in manufacturing.
- ISO 9001 Certification in Nepal: Elevate overall quality
management.
- ISO 15189:2022 Certification for Medical Laboratories in
Nepal: Elevate medical laboratory practices.
- ISO 17025 Certification for Testing and Calibration
Laboratories in Nepal: Ensure accurate testing and calibration.
- ISO 27001:2022 Certification for Information Security in
Nepal: Enhance information security practices.
- ISO 22000 Certification for Food Safety Management System in
Nepal: Ensure safe and quality food production.
- ISO 45001:2018 Certification for Occupational Health and
Safety in Nepal: Elevate health and safety standards.
- ISO 14001:2015 Certification for Environmental Management in
Nepal: Enhance environmental sustainability.
Our dedicated team is ready to provide your organization with customized solutions and expert assistance. If you have any queries or are ready to embark on your ISO certification journey, feel free to contact us at 9840525565 for a free consultation on our ISO certification services. Trust Quality Management System Nepal Pvt. Ltd. to be your partner in achieving excellence and compliance.