ISO 15189:2022 Medical laboratories — Requirements for quality and competence.
ISO 15189:2022 is an internationally recognized standard specifically designed for medical laboratories. Published by the International Organization for Standardization (ISO), this standard sets out the requirements for the quality and competence of medical laboratories, ensuring the provision of accurate and reliable laboratory results. The latest version, ISO 15189:2022, introduces updates and improvements to enhance laboratory operations and promote patient safety.
The Importance of ISO 15189
ISO 15189 holds immense significance for medical
laboratories and the healthcare industry as a whole. It provides a
comprehensive framework that guides laboratories in achieving consistent,
accurate, and reliable results. Here are some key reasons why ISO 15189 is
crucial:
- Enhanced
Patient Safety: Patient safety is paramount in healthcare. ISO 15189
emphasizes the importance of risk management, ensuring that laboratories
implement appropriate measures to identify and mitigate potential risks.
By adhering to the standard's guidelines, medical laboratories can
minimize errors, reduce the chances of misdiagnosis, and enhance patient
safety.
- Quality
Assurance: ISO 15189 establishes a robust quality management system (QMS)
that focuses on the continuous improvement of laboratory processes. The
standard outlines guidelines for document control, personnel competency,
equipment maintenance, and internal and external quality control. These
measures help maintain consistent quality and improve the overall
performance of medical laboratories.
- Reliable
and Accurate Results: Accurate laboratory results are crucial for
effective patient care and treatment decisions. ISO 15189 ensures that
laboratories implement standardized procedures, rigorous testing
methodologies, and proficiency testing to ensure the accuracy and
reliability of their results. This helps instill confidence in healthcare
professionals and patients, ultimately leading to better health outcomes.
- Global Recognition and Collaboration: ISO 15189 is internationally recognized and respected. By implementing this standard, medical laboratories can demonstrate their commitment to quality and competence. This recognition not only improves the laboratory's credibility within the local healthcare system but also facilitates international collaborations, exchange of information, and the harmonization of laboratory practices across borders.
ISO 15189 Benefits
The adoption of ISO 15189 brings several benefits to medical
laboratories and healthcare organizations:
- Compliance
with Regulatory Requirements: Many countries have incorporated ISO 15189
into their regulatory frameworks for medical laboratories. By complying
with the standard, laboratories can ensure they meet the necessary
regulatory requirements, thereby avoiding penalties and legal issues.
- Improved
Efficiency: ISO 15189 encourages laboratories to streamline their
processes and workflows, leading to increased efficiency. Clear guidelines
on equipment maintenance, calibration, and personnel training help optimize
resources, reduce waste, and enhance productivity.
- Continual
Improvement: ISO 15189 promotes a culture of continuous improvement within
medical laboratories. Regular internal audits, management reviews, and
corrective actions help identify areas for enhancement and ensure ongoing
progress in quality management.
- Customer Confidence: Patients, clinicians, and healthcare organizations place great trust in laboratories that adhere to ISO 15189. By implementing the standard, medical laboratories can instill confidence in their stakeholders, leading to stronger relationships, increased referrals, and improved reputation.
What is the difference between ISO 15189 and 17025?
ISO 15189 and ISO 17025 are both internationally recognized standards, but they have different scopes and applications within the field of laboratory testing and calibration. Here are the key differences between ISO 15189 and ISO 17025:
|
Aspect |
ISO
15189 |
ISO
17025 |
|
Scope |
Specifically
for medical laboratories. |
For
testing and calibration laboratories. |
|
Primary
Focus |
Quality
and competence in medical laboratories. |
Technical
competence in testing and calibration laboratories. |
|
Purpose |
Ensures
accuracy in medical testing and patient care. |
Ensures
accuracy in testing and calibration unrelated to health. |
|
Management
Requirements |
Contains
management requirements specific to clinical environments. |
Management
requirements more broadly applicable to any testing/calibration lab. |
|
Technical
Requirements |
Focused
on medical testing processes, including pre-examination, examination, and
post-examination processes. |
Focuses
on technical competence and results validity. Does not segment into pre,
during, and post like 15189. |
|
Patient
Involvement |
Directly
addresses patient involvement and care. |
Does
not cater specifically to patients; more focused on clients and service
recipients. |
|
Document
Control |
More
prescriptive about certain processes, including handling of complaints,
identification of samples, etc. |
More
general in approach, allowing labs to define their own processes in many
areas. |
|
Clinical
Decision-making |
Includes
guidance on the clinical interpretation of results. |
No
guidance on interpretation, only on obtaining valid results. |
Both standards highlight the importance of consistency,
accuracy, and validity in laboratory operations but cater to different types of
laboratories and have unique emphases based on their intended scopes.
ISO 15189 is specific to medical laboratories and emphasizes patient safety and quality in medical testing, while ISO 17025 is more general and applies to various types of testing and calibration laboratories, focusing on technical competence and quality assurance across different fields.
Controls and Requirements of ISO 15189 Certification
To achieve ISO 15189 certification, medical laboratories
must comply with a set of controls and requirements outlined in the standard.
These controls and requirements are designed to ensure the quality, competence,
and accuracy of laboratory testing processes. Here are some key controls and
requirements of ISO 15189 certification:
- Quality
Management System (QMS): Medical laboratories must establish and maintain
a documented QMS that defines policies, procedures, and processes to
achieve quality objectives. The QMS should cover all aspects of laboratory
operations, including sample collection, transportation, analysis, and
result reporting.
- Document
Control: Laboratories should have a robust document control system to
manage and control all relevant documents, including policies, procedures,
and work instructions. This ensures that all personnel have access to the
most up-to-date and accurate information.
- Personnel
Competency: ISO 15189 requires laboratories to have qualified and
competent personnel who possess the necessary education, training, and
experience to perform their assigned tasks. The standard emphasizes the
importance of ongoing competency assessment, training, and continuing
professional development for laboratory staff.
- Equipment
and Facilities: Laboratories must have appropriate equipment, instruments,
and facilities to perform accurate and reliable testing. ISO 15189
mandates that equipment is properly maintained, calibrated, and validated,
and that laboratories have adequate resources to support their testing
activities.
- Internal
and External Quality Control: Medical laboratories must implement robust
internal and external quality control programs. This includes regular
calibration and verification of equipment, participation in proficiency
testing programs, and monitoring of testing performance through the use of
control samples.
- Measurement
Uncertainty: ISO 15189 requires laboratories to estimate and report
measurement uncertainty for each measurement procedure. This helps ensure
that the reported results are accompanied by an indication of the degree
of uncertainty associated with the measurement.
- Risk
Management: The standard emphasizes the importance of risk management in
laboratory operations. Laboratories must identify and assess risks associated
with their testing processes and implement appropriate control measures to
mitigate those risks.
- Process
Improvement: ISO 15189 promotes a culture of continual improvement within
laboratories. Regular internal audits, management reviews, and corrective
actions are necessary to identify areas for improvement and implement
necessary changes.
- Proficiency
Testing: Laboratories should participate in external proficiency testing
programs to assess their performance and ensure the accuracy and
reliability of their testing results. This involves the comparison of
laboratory results with those of other laboratories using the same testing
methods.
- Customer
Satisfaction: ISO 15189 emphasizes the importance of meeting customer
requirements and ensuring customer satisfaction. Laboratories should have
mechanisms in place to receive and address customer feedback, complaints,
and suggestions.
By adhering to these controls and requirements, medical laboratories can demonstrate their compliance with ISO 15189 and ensure the provision of high-quality and reliable testing services.
What are the key requirements for achieving ISO 15189 accreditation?
The key requirements for ISO 15189 accreditation include but are not limited to:
- Documented quality management system (QMS) with defined policies and procedures.
- Competent personnel with appropriate qualifications and training.
- Validated and calibrated equipment and instruments for accurate testing.
- Proficiency testing and participation in external quality assessment schemes.
- Effective management of pre-examination, examination, and post-examination processes.
- Document control and record-keeping to track activities and results.
- Continuous improvement through data analysis, corrective actions, and preventive measures.
How can our medical laboratory implement ISO 15189?
To implement ISO 15189 in your medical laboratory, consider the following steps:
- Familiarize yourself with the ISO 15189 standard: Understand its requirements, clauses, and guidelines.
- Conduct a gap analysis: Evaluate your current laboratory practices against ISO 15189 requirements to identify areas that need improvement.
- Develop an implementation plan: Create a roadmap with specific actions and timelines to address the identified gaps.
- Train and engage staff: Provide training to laboratory personnel on ISO 15189, quality management principles, and their roles in implementation.
- Establish a quality management system: Develop and document procedures, policies, and processes to align with ISO 15189.
- Conduct internal audits: Regularly review and assess your laboratory's compliance with ISO 15189 to identify any non-conformities.
- Seek external accreditation: Engage an accredited certification body to conduct an independent assessment and grant ISO 15189 accreditation.
Is ISO 15189 Mandatory in Nepal?
Yes, ISO 15189 is mandatory for A grade labs in Nepal. The National Public Health Laboratory (NPHL) issued guidelines in 2022 stating that all A grade labs must be ISO 15189 accredited by 2024. This is in line with the government's efforts to improve the quality of healthcare in Nepal.
ISO 15189 is an international standard that specifies the requirements for quality and competence in medical laboratories. It is a rigorous standard that requires labs to have a robust quality management system in place. This ensures that the labs are providing accurate and reliable test results.
The mandatory accreditation of A grade labs with ISO 15189 is a positive step for the healthcare sector in Nepal. It will help to improve the quality of laboratory services and ensure that patients receive accurate and reliable test results.
If you are a medical laboratory in Nepal, I encourage you to consider pursuing ISO 15189 accreditation. It is a valuable investment that will help you to improve the quality of your services and provide your patients with the best possible care.
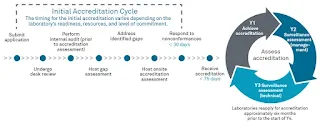
What is ISO 15189:2022 Accreditation?
The ISO 15189:2022 accreditation process typically involves
the following steps:
- Application:
The laboratory initiates the accreditation process by submitting an
application to an accredited accreditation body. The application includes
information about the laboratory's scope of testing and other relevant
details.
- Documentation
Review: The accreditation body reviews the laboratory's documentation,
including its quality manual, policies, procedures, and records, to assess
compliance with the requirements of ISO 15189:2022.
- On-site
Assessment: Accreditation assessors visit the laboratory to conduct an
on-site assessment. They evaluate the laboratory's facilities, equipment,
personnel competence, sample handling processes, quality control
procedures, and other aspects of the laboratory's operations.
- Assessment
Report: Based on the findings from the documentation review and on-site
assessment, the accreditation body prepares an assessment report that
identifies any non-conformities or areas for improvement.
- Corrective
Actions: The laboratory addresses any identified non-conformities or areas
for improvement and implements corrective actions within a specified
timeframe.
- Accreditation
Decision: The accreditation body reviews the assessment report and the
laboratory's corrective actions and makes a decision regarding accreditation.
If the laboratory meets the requirements of ISO 15189:2022, accreditation
is granted.
- Ongoing
Surveillance: Accredited laboratories are subject to regular surveillance
visits by the accreditation body to ensure continued compliance with the
standard. These visits may occur annually or at specified intervals.
ISO 15189:2022 accreditation provides recognition and validation of a laboratory's competence, quality management system, and commitment to providing accurate and reliable testing services. It enhances the laboratory's reputation, increases confidence in its results, and facilitates international recognition and collaborations.
Why is ISO 15189:2022 Accreditation important?
ISO 15189:2022 accreditation holds significant importance
for medical laboratories and the healthcare industry. It serves as a testament
to a laboratory's commitment to quality, competence, and patient safety. Here
are some key reasons why ISO 15189:2022 accreditation is important:
- Enhanced
Patient Safety: Accreditation to ISO 15189:2022 ensures that medical
laboratories adhere to rigorous quality standards and risk management
practices. This helps minimize errors and reduces the likelihood of
misdiagnoses or incorrect treatment decisions, ultimately leading to
improved patient safety and care.
- Reliable
and Accurate Results: ISO 15189:2022 emphasizes the importance of accurate
and reliable testing processes. Laboratories that are accredited to this
standard have implemented standardized procedures, quality control
measures, and ongoing proficiency testing to ensure the accuracy of their
results. This instills confidence in healthcare providers and patients in
the validity of the laboratory's findings.
- Global
Recognition: ISO 15189:2022 is an internationally recognized standard.
Laboratories accredited to this standard gain credibility not only within
their country but also on a global scale. This recognition facilitates
international collaborations, data exchange, and harmonization of laboratory
practices, allowing laboratories to be part of a larger network of trusted
providers.
- Compliance
with Regulatory Requirements: In some countries, ISO 15189:2022
accreditation may be adopted as part of the regulatory framework for
medical laboratories. Achieving accreditation ensures that the laboratory
meets the necessary regulatory requirements, avoiding potential penalties
and legal issues.
- Continual
Improvement: The accreditation process involves ongoing assessments and
surveillance visits by the accreditation body. This promotes a culture of
continuous improvement within the laboratory, leading to enhanced
efficiency, better resource utilization, and improved quality of services
over time.
- Strengthened
Customer Confidence: ISO 15189:2022 accreditation provides assurance to
healthcare providers, patients, and other stakeholders that the laboratory
operates with a focus on quality and competence. This enhances customer
confidence and fosters stronger relationships between the laboratory and
its clients.
- Competitive
Advantage: Accreditation to ISO 15189:2022 sets accredited laboratories
apart from non-accredited ones, creating a competitive advantage in the
market. Healthcare providers and patients are more likely to choose
accredited laboratories for their testing needs due to the assurance of
quality and reliability.
- Facilitates
Continuous Learning: The accreditation process often involves engagement
with external assessors who bring valuable insights and best practices
from other accredited laboratories. This exposure fosters a culture of
continuous learning and knowledge exchange within the laboratory.
ISO 15189:2022 accreditation is important because it demonstrates a laboratory's commitment to excellence, patient safety, and quality management. It provides a competitive edge, strengthens customer confidence, and opens doors to international collaborations, ultimately benefiting both the laboratory and the healthcare community it serves.
How can ISO 15189:2022 Accreditation be obtained?
Obtaining ISO 15189:2022 accreditation involves a series of
steps and processes. Here is a general overview of how the accreditation can be
obtained:
- Familiarize
Yourself with the Standard: Read and understand the requirements outlined
in ISO 15189:2022. This will help you assess your laboratory's current
practices and identify any gaps that need to be addressed.
- Internal
Gap Analysis: Conduct an internal gap analysis to compare your
laboratory's existing processes and procedures against the requirements of
ISO 15189:2022. Identify areas where your laboratory needs to make
improvements or implement new practices to meet the standard's criteria.
- Develop
and Implement a Quality Management System (QMS): Establish a comprehensive
QMS that incorporates the necessary policies, procedures, and processes to
meet the requirements of ISO 15189:2022. Document your QMS and ensure that
it is effectively communicated and implemented throughout the laboratory.
- Training
and Competency Assessment: Ensure that your laboratory staff receive
appropriate training and have the necessary competencies to perform their
assigned tasks. Establish processes for ongoing competency assessment and
provide opportunities for professional development and training.
- Conduct
Internal Audits: Perform regular internal audits to assess the
effectiveness of your QMS and identify areas for improvement. Correct any
non-conformities or deficiencies that are identified during the audit
process.
- External
Assessment: Engage an accredited external assessment body or accreditation
body to conduct an external assessment of your laboratory. This assessment
will involve a thorough review of your documentation, processes,
facilities, and staff competencies to ensure compliance with ISO
15189:2022.
- Corrective
Actions and Improvements: Address any findings or non-conformities
identified during the external assessment. Implement corrective actions
and make necessary improvements to align with the requirements of the
standard.
- Application
and Evaluation: Submit an application for ISO 15189:2022 accreditation to
an accredited accreditation body. The accreditation body will review your
documentation and assessment reports and evaluate your laboratory's
compliance with the standard.
- Accreditation
Decision: Based on the evaluation, the accreditation body will make a
decision on whether to grant ISO 15189:2022 accreditation to your
laboratory. If successful, you will receive a formal accreditation
certificate.
- Ongoing
Surveillance and Reassessment: Maintain compliance with the standard and
undergo regular surveillance visits by the accreditation body to ensure
that your laboratory continues to meet the requirements of ISO 15189:2022.
Accreditation is typically reassessed every few years to ensure ongoing
compliance.
It is important to note that the specific process and requirements for ISO 15189:2022 accreditation may vary depending on the accreditation body and country-specific regulations. Therefore, it is recommended to consult with the appropriate accreditation body or seek the guidance of a consultant experienced in ISO accreditation processes to ensure a smooth and successful accreditation journey. Contact 9840525565 to learn more about accreditation process.
How can ISO 15189 benefit our laboratory and its stakeholders?
ISO 15189 accreditation offers several benefits:
- Enhanced confidence: Stakeholders gain trust in the laboratory's competence and reliability of test results.
- Improved patient safety: Accurate and reliable testing reduces the risk of misdiagnoses and medical errors.
- international recognition: ISO 15189 is a globally recognized standard, facilitating international cooperation and collaboration.
- Operational efficiency: The implementation of a quality management system improves laboratory processes and performance.
- Compliance with regulations: ISO 15189 accreditation ensures adherence to regulatory requirements.
Conclusion:
Obtaining ISO 15189:2022 accreditation involves a thorough and systematic process, including an internal gap analysis, the development and implementation of a robust quality management system, regular internal audits, and ultimately an external assessment by an accredited accreditation body.
Frequently Asked Questions:
What are the ISO standards?
ISO standards are a set of internationally recognized guidelines and specifications developed by the International Organization for Standardization (ISO) to ensure consistency, safety, and quality across various industries and sectors. These standards cover a wide range of topics, such as quality management, environmental management, information security, and more.
What is quality management system ISO 15189?
ISO 15189 is a specific ISO standard that pertains to medical laboratories. It defines the requirements for a quality management system (QMS) in laboratory medicine, focusing on the competence and capability of the laboratory to produce reliable test results and ensure patient safety.
What is ISO Certification?
ISO Certification is a seal of approval from a third-party
body that verifies a company adheres to one of the internationally recognized
ISO management systems.
Why is ISO Certification important in Nepal?
ISO Certification in Nepal helps businesses enhance their
processes, boost customer confidence, achieve competitive advantage, and access
international markets. It also ensures that companies in Nepal are on par with
global standards.
How can "Quality Management System Nepal Pvt. Ltd." assist me in obtaining ISO Certification?
We provide comprehensive services including training,
documentation support, gap analysis, implementation assistance, and audit
support to ensure a smooth and efficient certification process for your
organization.
How long does it take to get ISO certified?
The time frame can vary depending on the size of the
organization and the ISO standard pursued. Typically, it can take a few months
up to a year.
Is the certification valid internationally?
Yes, ISO Certifications are internationally recognized and
accepted.
How often are surveillance audits conducted post-certification?
Typically,
surveillance audits are conducted annually to ensure ongoing compliance with
the standard.
What is the validity period of the ISO certificate?
An ISO certificate is generally valid for three years,
subject to satisfactory surveillance audits.
Can small businesses also get ISO certified?
Absolutely! ISO
certification is suitable for both small and large businesses. The focus is on
the quality of processes, not the size of the business.
How do we maintain our ISO certification?
You can maintain your certification by continually adhering
to the ISO standards, conducting regular internal audits, addressing any
non-conformities, and undergoing the required surveillance audits.
What are the advantages of getting ISO Certification in Nepal through "Quality Management System Nepal Pvt. Ltd."
Our local presence and expertise ensure that you receive
tailored assistance, understanding of the Nepali context, efficient service,
and continuous support throughout the certification journey.
What is the purpose of ISO 15189?
The purpose of ISO 15189 is to ensure that medical laboratories establish and maintain a quality management system that provides accurate and reliable testing services. It emphasizes the importance of competence, consistency, and continual improvement in laboratory operations to enhance patient care and safety.
A practical guide to ISO 15189 in laboratory medicine?
"A practical guide to ISO 15189 in laboratory medicine" would likely be a comprehensive document or book that provides practical insights, instructions, and examples to assist medical laboratories in understanding and implementing ISO 15189 requirements effectively. It could cover topics such as documentation, process improvement, competency assessment, and internal auditing, tailored specifically for laboratory settings.
What changes are needed in our current practices to comply with ISO 15189?
To comply with ISO 15189, your laboratory might need
to make the following changes:
· Adopt a quality-focused culture: Emphasize
the importance of quality and patient safety among all staff members.
· Review and revise procedures: Ensure that laboratory processes align with ISO requirements.
· Enhance training and competency: Provide
staff with appropriate training and ensure their competency for specific tasks.
· Improve documentation: Implement
effective record-keeping practices to maintain traceability and accountability.
· Implement internal audits: Regularly
assess your laboratory's compliance and address any non-conformities.
· Participate in external assessments: Engage with accredited certification bodies for the formal ISO 15189 accreditation process
In order to maintain a seamless and efficient ISO certification process, partnering with a trusted ISO consultant is crucial. At Quality Management System Nepal Pvt. Ltd., we are committed to providing your organization with expert guidance and support, ensuring a cost-effective and successful ISO implementation journey. As the leading ISO System Certification body in Nepal, we offer a comprehensive range of certification services tailored to your organization's needs.
Our dedicated team is ready to provide your organization
with customized solutions and expert assistance. If you have any queries or are
ready to embark on your ISO certification journey, feel free to contact us at
9840525565 for a free consultation on our ISO certification services. Trust Quality Management System Nepal Pvt. Ltd. to be your partner in achieving excellence and
compliance.